The Story of the Atom
Atoms are the building blocks of everything you see around. Even though scientists have discovered that the atom is made up of smaller subatomic particles, the atom is the smallest part of matter that represents a particular element. This article is about the major breakthroughs about the discovery of the atom.
“Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible. That is, there must be some point beyond which we cannot go in the division of matter. I have chosen the word “atom” to signify these ultimate particles.” — John Dalton

John Dalton, a English chemist, physicist, and meteorologist, defined the atom as the smallest indivisible particle. Though we know today that they are composed of three fundamental particles: proton, neutrons and electrons, his contributions to atomic theory were crucial and revolutionary in his time.
Despite the fact that not all of them are correct, but the essence of his theories remain valid. Dalton’s atomic theory was generally accepted because it explained the laws of conservation of mass, definite proportions, multiple proportions, along with other observations.
The Law of Conservation of Mass states that the net change in mass of the reactants and products before and after a chemical reaction is zero. This means mass can neither be created nor destroyed. In other words, the total mass in a chemical reaction remains constant.
The Law of Definite Proportions states that a given chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation.
The Law of Multiple Proportions states that if two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.
Although exceptions to Dalton’s theory are now known, his theory has endured reasonably well, with modifications, throughout the years. He stated the following postulates:
- All matter consists of indivisible particles called atoms.
- Atoms of the same element are similar in shape and mass, but differ from the atoms of other elements.
- Atoms cannot be created or destroyed.
- Atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms.
- Atoms of same element can combine in more than one ratio to form two or more compounds.
- The atom is the smallest unit of matter that can take part in a chemical reaction.
The drawbacks of his model as we know today include:
- An atom can be divided further into different subatomic particles.
- It could not explain the existence of isotopes.The second postulate is wrong as some atoms of the same element vary in mass.(Cl has isotopes of 35 and 37)
- It could not explain the existence of isobars. Different elements can have the same mass. (Ca and Ar have the same mass of 40 amu.)
- It could not explain the existence of allotropes. (Graphite and Diamond are both made of C atoms but they have differing properties.)
- Atoms can be destroyed or broken down by the fission process in the nuclear reactor.
Based on his observations, Dalton proposed the billiard ball model. He defined an atom to be a ball like structure, as the concept of subatomic particles was unknown then. He also was on the first scientists to try and symbolize atoms, and he became one of the first scientists to assign such symbols. He gave a specific symbol to each atom.
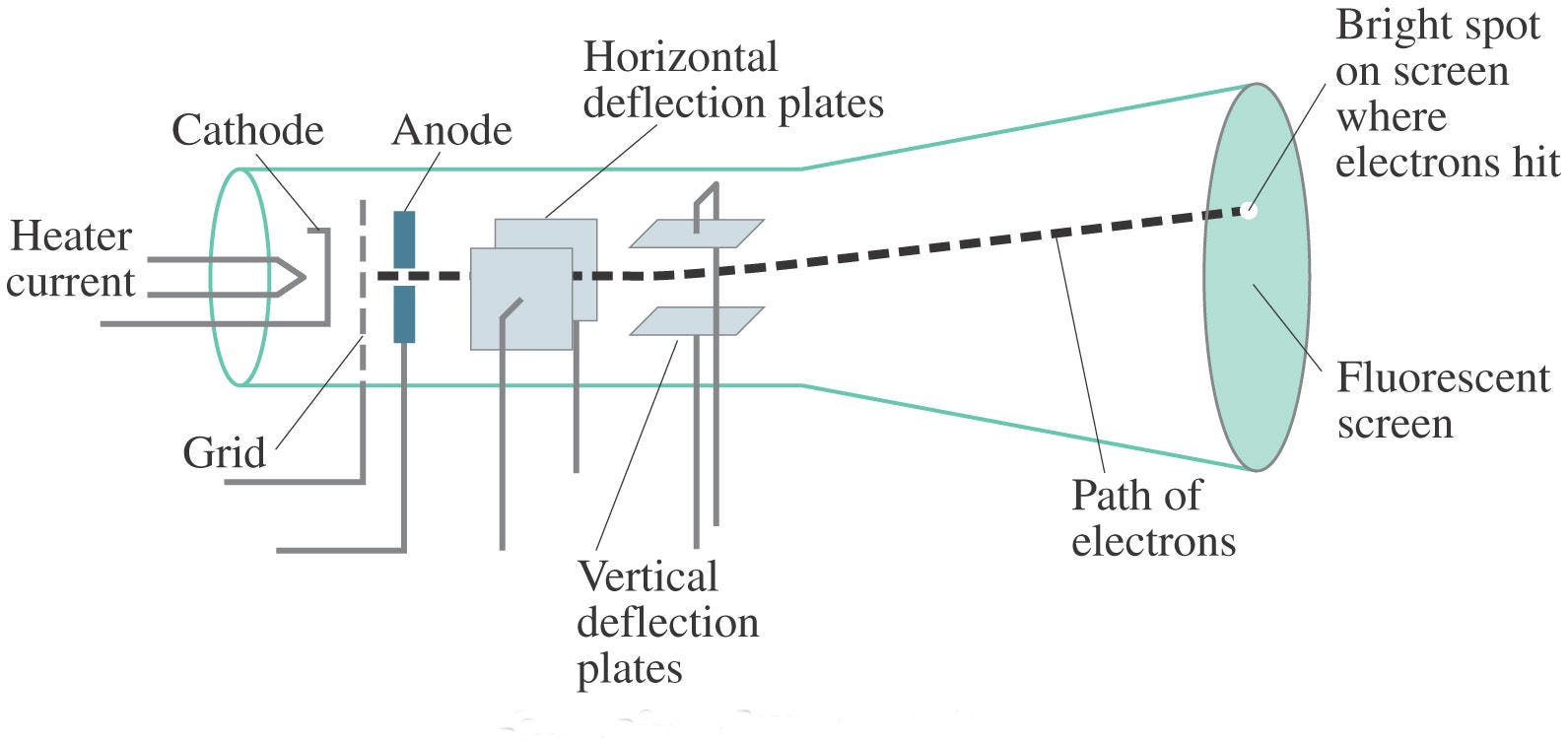
In 1896, the British physicist J. J. Thomson performed experiments demonstrating that cathode rays were unique particles, rather than waves, atoms or molecules, as was believed earlier. Thomson made good estimates of both the charge e and the mass m, finding that cathode ray particles had perhaps one thousandth the mass of hydrogen, the least massive ion known. He showed that their charge to mass ratio (e/m) was independent of cathode material.
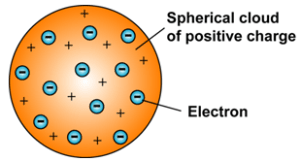
He then concluded and laid the fundamental concept of the structure of the atom. He referred to it as the plum pudding model.
He proposed the following two key points:
- An atom consists of a positively charged sphere, and the negatively charged particles are embedded in it.
- The negatively charged particles and the positively charged particles are equal in magnitude, i.e. an atom is electrically neutral.
However, a Japanese scientist named Hantaro Nagaoka rejected Thomson’s model on the grounds that opposing charges cannot penetrate each other. He proposed instead that electrons orbit the positive charge like the rings around Saturn.
In 1909, the famous gold foil experiment performed by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford, a former student of Thomson disproved the Thomson Plum Pudding model. This gold foil experiment was interpreted by Ernest Rutherford in 1911 to suggest that there is a very small nucleus of the atom that contains a very high positive charge. His conclusions led him to propose the Rutherford model of the atom which will be discussed in the next section.
The gold foil experiment Rutherford designed was intended to further explore the structure of the atom. The experiment begins by shooting alpha particles with at high speeds towards a thin foil of gold. If the plum pudding model was accurate, the alpha particles would all have been deflected by, at most, a few degrees. To their disbelief, only some of the alpha particles passed through as expected, although many others were deflected at small angles while others were reflected back to the alpha source. They recorded the location of the alpha particle “strikes” on a fluorescent screen as they passed through the foil. Measuring the pattern of scattered particles was expected to provide information about the distribution of charge within the atom.
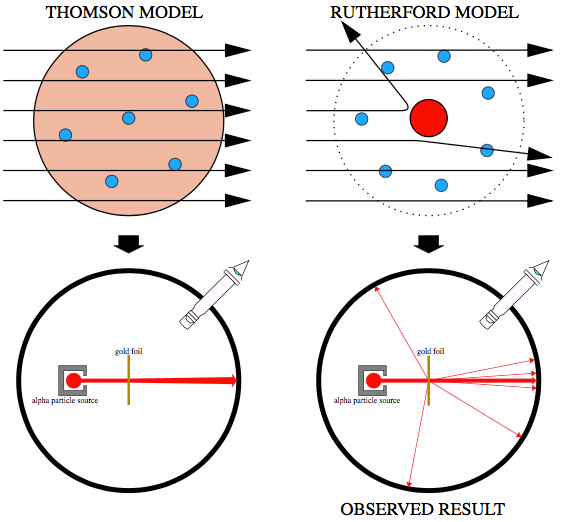
From this it can be concluded that there is a small, dense positive charge in the center of the atom while negatively charged electrons surround it. This central volume also contained the bulk of the atom’s mass. This region would later be named the ‘nucleus’. This was thought to be Rutherford’s greatest scientific work.
“We must be clear that when it comes to atoms, language can only be used as in poetry.” — Niels Bohr
Niels Bohr, a Danish physicist, made further use of his Rutherford’s planetary model of the atom.

The major flaw with the old atomic model was that the laws of classical mechanics predict that the electrons should release electromagnetic radiation when orbiting a nucleus. This atom model is disastrous, because it predicts that all atoms are unstable, as the electrons would gradually spiral inwards, falling into the nucleus.
To overcome this difficulty, Bohr proposed, in 1913, what is now called the Bohr model of the atom. He used quantum theory to explain the behavior of electrons around a nucleus. He suggested that electrons could only have certain classical motions:
- Electrons in atoms orbit the nucleus.
- The electrons can only orbit stably, without radiating, in certain orbits (called by Bohr the “stationary orbits”): at a certain discrete set of distances from the nucleus. These orbits are associated with definite energies and are also called energy shells or energy levels. In these orbits, the electron’s acceleration does not result in radiation and energy loss as required by classical electrodynamics.
This meant that electrons only existed in ‘fixed’ orbits or energy levels where they did not radiate energy. The energy levels could be assigned quantum numbers. Energy would only be radiated if the electrons moved between a higher energy orbit and a lower energy orbit. Bohr’s application of his theory to hydrogen atoms matched physical observations. His predictions of the wavelengths of light that hydrogen atoms should release when electrons moved between orbits also matched physical observations.
- Bohr’s model only explains the spectra of species that have a single electron, such as the hydrogen atom.
- Bohr’s theory predicts the origin of only one spectral line from an electron between any two given energy states. Under a spectroscope of strong resolution, a single line is found to split into a number of very closely related lines. Bohr’s theory could not explain this multiple or fine structure of spectral lines. The appearance of the several lines implies that there are several sub energy levels of nearly similar energy for each principal quantum number, n. This necessitates the existence of new quantum numbers.
- It does not explain the splitting of spectral lines under the influence of a magnetic field (the Zeeman effect) or under the influence of an electric field (the Stark effect).
- The pictorial concept of electrons jumping from one orbit to another orbit is not justified because of the uncertainty in their positions and velocities.
“Anyone who is not shocked by quantum theory has not understood it.” — Niels Bohr
An Austrian physicist, Erwin Schrödinger published the Quantum Mechanical Model. Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position.
Unlike the Bohr model, the quantum mechanical model does not define the exact path of an electron, but rather, predicts the odds of the location of the electron. This model can be portrayed as a nucleus surrounded by an electron cloud. Where the cloud is most dense, the probability of finding the electron is greatest, and conversely, the electron is less likely to be in a less dense area of the cloud. Thus, this model introduced the concept of sub-energy levels. An atomic orbital is defined as the region within an atom that encloses where the electron is likely to be 90% of the time.
This is widely accepted as the most accurate model of the atom.
Maybe in another 10 years, a new model of the atom will be published. Did you enjoy this article? Click the 👏 to let me know! Thanks for reading!

/image%2F3362239%2F20190507%2Fob_bdec05_2.gif)


/https%3A%2F%2Fgame-tool.rocks%2Fimg%2Fh3.png)
